Group 17 Periodic Table
Interactive periodic table of elements - your complete guide to the elements including definition mass names of each chemical in the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.

What Is The Periodic Table Of The Elements A Plus Topper Https Www Aplustopper Com His Periodic Table Periodic Table Of The Elements Relative Atomic Mass
This interactive periodic table of element groups arranges the chemical elements according to periodicity or common properties.

. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Groups Groups are the vertical columns in a periodic table. Non-Metallic Character of The Elements.
Periodic Table or Periodic Chart of Elements showing Gases. Noble gases group. As we have seen in post 28 the sequence of filling the orbitals is such that the energy of the ns shell is always less than n-1 d shell So the electrons fill the ns shell first and later they enter the penultimate n-1d orbital.
Be H 2 O 4 2. The chemical elements of the same group had similar properties. Across a Group As we move top to bottom in a group of the periodic table the metallic character of elements increases.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. Store and use at 20 4C. Fluorine F Chlorine Cl Bromine Br Iodine I Astatine At Tennessine Ts For detailed information on halogens read the main article on Halogen elements of Periodic table.
The elements included in halogen group are. A periodic table is divided into groups columns where elements with each group behave similarly while bonding with other elements. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
The atomic number of each element increases by one reading from left to right. Example Elements of 2 nd period. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all.
The elements in a group do not have the same atomic number. Skip to main content. By mass oxygen is the.
All the elements in the transition series and the f block as well as the elements of the. Science Tech Math Science Math Social Sciences Computer Science Animals Nature Humanities History Culture Visual Arts Literature English Geography Philosophy Issues Languages English as a Second Language. A vertical column in the periodic table.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Do not return portions removed. In the long form of the periodic table there are 18 groups.
Therefore it is easier to remove an electron from lower in a group. This periodic table guide breaks down the elements name atomic weight and oxidation state. 2In these elements the penultimaten-1 d-orbital is filled with electrons.
This is because with 7 valence electrons. There are different regions in the periodic table that are called periodic table blocks as they are named according to the subshell of the last electron of the atom. It is a member of the chalcogen group on the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.
Interactive periodic table of the chemical elements. Period A horizontal row in the periodic table. Electrons from the attractive forces of the nucleus.
On the opposite end of the spectrum group 17 elements have very high ionization energies. Noble gases group is the last group group 18 on the. Characteristics of Periods in a Periodic Table.
For example elements on the left-hand side of the periodic table are generally metals. The next six elements on the periodic table are expected to be the last main-group elements in their period. Group 2 Period 2.
As you can see on the graph the noble gases have the highest ionization energies and. Alkali Metals Alkaline Earth Metals. Keep tightly sealed when not in use.
Group 1 is on the left side of the periodic table whereas group 18 is on the right. Chemical Form in Solution. Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure.
Halogen group is the group 17 on the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Do not pipet from container.
It serves as a great tool for solving chemistry problems. The Periodic Table Trend on a Graph. 1These elements are found in groups 3 to 12 in the periodic table.
And periods rows where elements in one period have same. The periodic table that we use today contains seven periods and 18 groups. This design lets you quickly find an elements symbol atomic number and atomic mass.
Block Elements are organised into blocks by the orbital type in which the outer electrons are found. Get essential facts about the first 20 elements all in one convenient place including the name atomic number atomic mass element symbol group and electron configurationIf you need detailed facts about these elements or any of the higher numbered ones start with the clickable periodic table. Beyond element 172 another long transition series like the superactinides should begin filling at least the 6g 7f and 8d shells with 10s 10p 12 and 6h 112 too high in energy to.
An easy to use guide to the elements of the periodic table including their history discovery physical and chemical. Oxygen is a colourless odourless reactive gas the chemical element of atomic number 8 and the life-supporting component of the air. The first group is the alkali metals.
Award winning periodic table by relative atomic mass with user-friendly element data and facts. Periodic Table of the Elements GROUPS Alkali Metals Alkaline Earth Metals Blocks Gases stp Halogens LanthanidesActinides Liquids stp Main Group Metalloids Metals Noble Gases Non. The Periodic Table provides the names atomic numbers symbols and atomic weights of known elements.
An up-to-date periodic table with detailed but easy to understand information. Around 45ths of the periodic table elements are metals. Definition mass chemical names.
The second is called the alkali earth metals the d block of elements are known as the transition series. The elements of a Mendeleevs table were arranged in rows called periods and columns called groups. Properties history name origin facts applications isotopes electronic configuation crystal structure.
Home About This Site Comments Help Links Window Version. Across a Period As we move left to right across a period in the periodic table the non-metallic character of elements increases. For instance group 17 is called the halogens and the group 18 is the noble gases.
The table can also give you information on chemical properties.

Tabel Periodik Kimia Bilangan Oksidasi

Actinoid Element Chemical Element Group Periodic Table Of The Elements Periodic Table Sms Language

Physical And Chemical Properties Of Group 17 Elements A Plus Topper Chemicalpropertiesgroup17ele Physical And Chemical Properties Chemical Property Physics
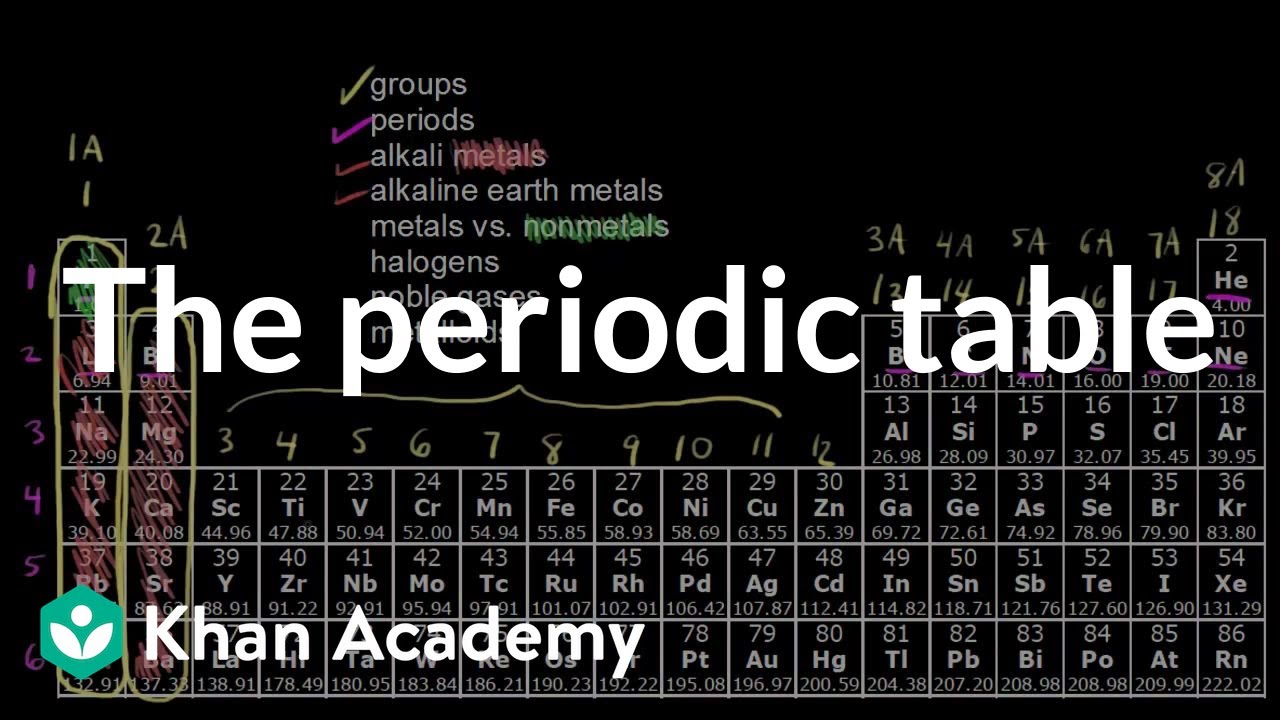
Definitions Of Groups Periods Alkali Metals Alkaline Earth Metals Halogens And Noble Gases How Metals Non Meta Periodic Table Chemistry Chemistry Basics

Physical Chemical Properties Group 17 Elements 4 Physical And Chemical Properties Chemical Property Chemical

Comments
Post a Comment